|
Size: 7294
Comment:
|
Size: 7316
Comment:
|
| Deletions are marked like this. | Additions are marked like this. |
| Line 2: | Line 2: |
| <<TableOfContents(3)>> |
Contents
Il documento al sequente link riporta le regole di rendicontazione documentano la Decision of 14 November 2012 establishing a common format for the submission of the information pursuant to Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes (notified under document C(2012) 8064) (Text with EEA relevance) (2012/707/EU) Link Eur-Lex
La figura sottostante mostra lo schema di inserimento dei dati nel sistema informativo della Banca dati della sperimentazione animale.
![]()
Il seguente materiale è tratto dal Manuale Input data into ALLURE: è indicato il prospetto riepilogativo contenente il significato di tutti i campi. Sono inoltre definite le regole di obbligatorietà (mandatory field) e dipendenza (dependent field) fra campi della rendicontazione Allure. Qualora i dati inseriti non corrispondano ai criteri individuati da ALLURES, il sistema presenterà dei messaggi di errore come mostrato di seguito.
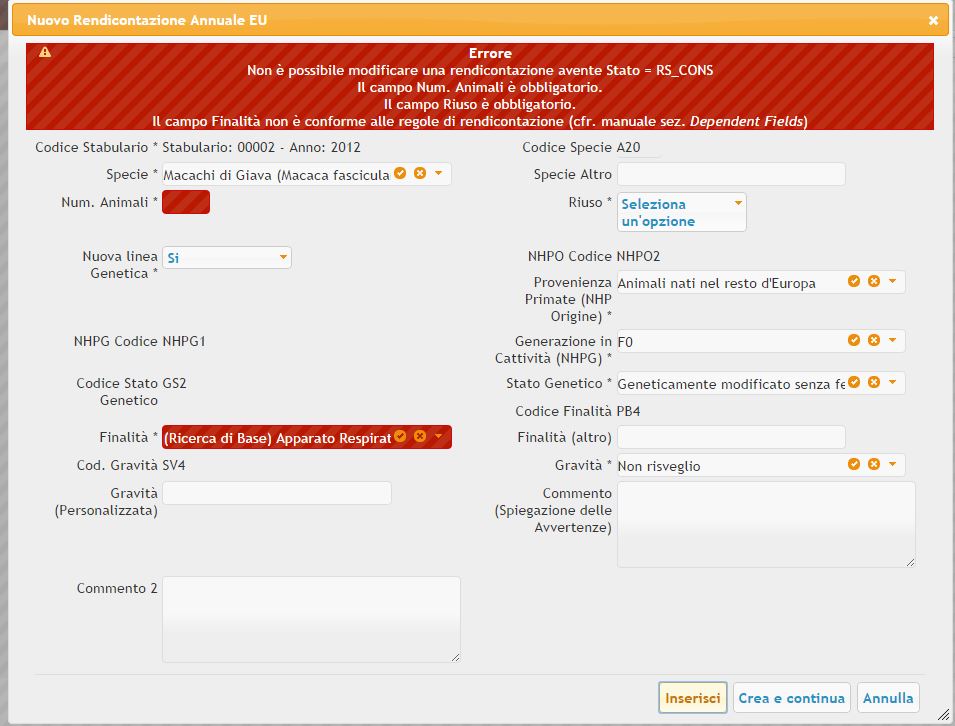
Animal Usage Data Entry
Animal Species: Mandatory field. The species of animal used in the research/experiment. Only one element can be selected from the list found in Annex 1.
Specify other: Dependent field. If you choose, in the previous field, other animals (e.g. other birds or other mammals) please specify what species was used exactly. If you have chosen a species that needs specification, species field would change its format.
[A3]
Guinea-Pigs (Cavia porcellus)
[A29]
Other Birds (altro Aves)
Number of animals: Mandatory field. Indicate here how many animals are used in the research/experiment.
Re-use: Mandatory field. Dropdown combo box with yes/no question. Indicate here if animals were used before in other research/or another experiment or not.
Place of birth: Dependent field. Dropdown combo box with a list of values found in the annexed list. Indicate here where animals were born.
NHP Source: Dependent field. Full name is Non-Human Primate Source. Dropdown combo box with a list of values. Indicate here where primates were born and if they come from a registered breeder.
NHP Generation: Dependent field. Full name is Non-Human Primate Generation. Dropdown combo box that contains different types of generation for an animal. Indicate here generation that applies to the primates.
Genetic status: Mandatory field. Dropdown combo box with different types of genetic alterations. Choose if animals were genetically altered or not in the research/experiment and alteration type.
Creation of new GL: Mandatory field. Full name is Creation of new Genetic Line. Dropdown combo box with yes/no question. Choose whether a new genetic line was created in the research/experiment or not.
Purpose: Mandatory field. Dropdown combo box with different types of research/experiment. Indicate here the reason for the research/experiment and the area of investigation. Please note that in cases where a Member State chooses to use the same excel form for the submission of additional national reporting requirements which go beyond that of the EU reporting, these purpose fields will be added in comment columns at the end.
Specify Other: Dependent field. If you choose, in the previous field, other purpose (e.g. other basic research or other human disorders) you have to specify what the purpose was exactly. If you have chosen a purpose that needs specification, the purpose field will change its format.
Testing by legislation: Dependent field. Dropdown combo box with different legislative instruments. Indicate here under which specific legislation the use of animals is included.
Specify Other: Dependent field. If you choose, in the previous field, other (last one: LT10) you have to specify the legislation that applies. If you have chosen "other", the testing by legislation field would change its format.
Legislative Requirements: Dependent field. Dropdown combo box with different legislative sources. Indicate here on the basis of which legislation the use of animals is carried out by origin of the legislation.
Severity: Mandatory field. Dropdown combo box with different research/experiment degree of severity. Indicate here the actual severity that the animal experienced during the research/experiment.
Custom severity: Optional field for those MS which require further breakdown of the standard EU severity categories. It is important to note that even if the Custom severity is used, the EU Severity field is mandatory and should be completed.
Comments 1: Optional field. Comments are optional fields that allow a data entry to contain additional relevant information. These comments will not be automatically processed by the application, but are visible to an operator handling the submission file. The comment can be used for whatever purpose they can be judged useful: justification of input data, etc.
Comments 2: Optional field. Additional comment field.
Dependent Fields
As indicated, there are some different types of fields: optional, mandatory and dependent. Mandatory and optional are clear: they correspond to, respectively, mandatory fields without which the submission is invalid, and optional fields that are non-compulsory but useful for a particular purpose.
Dependent fields could be mandatory or blank fields depending on value/s from another field/s. There are 5 dependent fields in the submission, they are: Place of birth, NHP Source and NHP Generation depend on Species and Re-use.
Place of birth must be completed if and only if Re-use is set to "No" and, in Species, is selected a non-NHP (values outside the range A19~A25). On the other hand, NHP Source and NHP Generation must be completed only if NHP is selected in Species (values inside the range A19~A25). For better understanding, below you can see a dependency diagram and table for these fields.
The next 2 dependent fields are Testing by Legislation and Legislative Requirements. These 2 fields depend on the Purpose field. If the Purpose value does not start with code PR, leave these 2 dependent fields blank. If Purpose value starts with code PR, you have to select values for these 2 fields from the dropdown list.
Finally, there is one special field: Purpose. Purpose is mandatory in any case but its list can change depending on your selection in Creation of a new GL. If you choose "Yes" in Creation of a new GL you can only choose in Purpose field a value inside "Basic research purposes" (PB) and "Translational and applied research purposes" (PT). In other cases you can choose any option from the complete list of purposes. However, do not worry about this, because excel will create a purpose list dynamically. So, you will not be able to introduce a bad value for purpose.
