Contents
Gestione delle Rendicontazione Dati Statistici ALURES
La compilazione della rendicontazione nell'ambiente DECLARE/ALURES (di seguito, ALURES) segue le regole riportate nei documenti ufficiali Eur-Lex.
In particolare il sistema informativo si basa sulle regole definite nell'allegato II del documento Commission Implementing Decision of 14 November 2012 establishing a common format for the submission of the information pursuant to Directive 2010/63/EU of the European Parliament and of the Council on the protection of animals used for scientific purposes (notified under document C(2012) 8064) (Text with EEA relevance) (2012/707/EU) Documento 14 November 2012 - Eur-Lex e nelle successive modifiche riportate nel documento 02012D0707-20140115 Documento 20140115 Eur-Lex.
Inoltre, il sistema recepisce le norme del decreto legislativo di Attuazione della direttiva 2010/63/UE sulla protezione degli animali utilizzati a fini scientifici (Dlgs 26 del 2014).
La figura sottostante mostra lo schema di inserimento dei dati nel sistema informativo della Banca dati della sperimentazione animale. A questo link è disponibile l'elenco dei valori codificati dal sistema ALURES ammessi per la rendicontazione (in accordo all' ARTICOLO 54(1) della DIRETTIVA 2010/63/EU).
Il seguente materiale è tratto dal Manuale Input data into ALURES: è indicato il prospetto riepilogativo contenente il significato di tutti i campi. Sono inoltre definite le regole di obbligatorietà (mandatory field) e dipendenza (dependent field) fra campi della rendicontazione ALURES. Qualora i dati inseriti non corrispondano ai criteri individuati da ALURES, il sistema presenterà dei messaggi di errore come mostrato di seguito.
Modalità Generali di Rendicontazione
Non è necessario rendicontare per singolo animale. Le voci di rendicontazione possono essere inserite aggregando i dati secondo una permutazione dei vari attributi proposti (specie/riuso/genotipo/fenotipo/finalità/gravità//legislazione, ecc). In questo modo, tutti gli animali con le stesse caratteristiche vanno a costituire una riga di rendicontazione e dunque rientrano nella stessa "voce", anche se facenti parte di protocolli di ricerca differenti (ai fine della rendicontazione non è richiesto alcun riferimento al singolo progetto).
Un tutorial per la finalizzazione di una rendicontazione annuale può essere seguito al seguente link ESEMPI di RENDICONTAZIONE. Di seguito vengono riportati i significati dei valori statistici, da specificare voce per voce.
Animal Usage Data Entry
Animal Species: Mandatory field. The species of animal used in the research/experiment. Only one element can be selected from the list found in Annex 1.
[A3]
Guinea-Pigs (Cavia porcellus)
[A29]
Other Birds (altro Aves)
(Elenco Specie Animali ).
Specify other: Dependent field. If you choose, in the previous field, other animals (e.g. other birds or other mammals) please specify what species was used exactly. If you have chosen a species that needs specification, species field would change its format.
Number of animals: Mandatory field. Indicate here how many animals are used in the research/experiment.
Re-use: Mandatory field. Dropdown combo box with yes/no question. Indicate here if animals were used before in other research/or another experiment or not.
Place of birth: Dependent field. Dropdown combo box with a list of values found in the annexed list. Indicate here where animals were born.
NHP Source: Dependent field. Full name is Non-Human Primate Source. Dropdown combo box with a list of values. Indicate here where primates were born and if they come from a registered breeder.
NHP Generation: Dependent field. Full name is Non-Human Primate Generation. Dropdown combo box that contains different types of generation for an animal. Indicate here generation that applies to the primates.
Genetic status: Mandatory field. Dropdown combo box with different types of genetic alterations. Choose if animals were genetically altered or not in the research/experiment and alteration type.
Creation of new GL: Mandatory field. Full name is Creation of new Genetic Line. Dropdown combo box with yes/no question. Choose whether a new genetic line was created in the research/experiment or not.
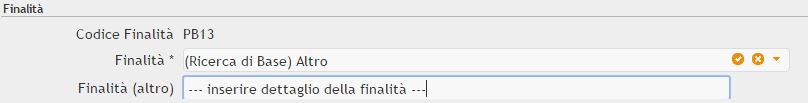
Purpose: Mandatory field. Dropdown combo box with different types of research/experiment. Indicate here the reason for the research/experiment and the area of investigation. Please note that in cases where a Member State chooses to use the same excel form for the submission of additional national reporting requirements which go beyond that of the EU reporting, these purpose fields will be added in comment columns at the end.
(Elenco Finalità ).
Specify Other: Dependent field. If you choose, in the previous field, other purpose (e.g. other basic research or other human disorders) you have to specify what the purpose was exactly. If you have chosen a purpose that needs specification, the purpose field will change its format.
Testing by legislation: Dependent field. Dropdown combo box with different legislative instruments. Indicate here under which specific legislation the use of animals is included.
Specify Other: Dependent field. If you choose, in the previous field, other (last one: LT10) you have to specify the legislation that applies. If you have chosen "other", the testing by legislation field would change its format.
Legislative Requirements: Dependent field. Dropdown combo box with different legislative sources. Indicate here on the basis of which legislation the use of animals is carried out by origin of the legislation.
Severity: Mandatory field. Dropdown combo box with different research/experiment degree of severity. Indicate here the actual severity that the animal experienced during the research/experiment.
Custom severity: Optional field for those MS which require further breakdown of the standard EU severity categories. It is important to note that even if the Custom severity is used, the EU Severity field is mandatory and should be completed.
Comments 1: Optional field. Comments are optional fields that allow a data entry to contain additional relevant information. These comments will not be automatically processed by the application, but are visible to an operator handling the submission file. The comment can be used for whatever purpose they can be judged useful: justification of input data, etc.
Comments 2: Optional field. Additional comment field.
Si noti che le scelte che indicano altro (es. specie o finalità) andranno ulteriormente specificate con un ulteriore campo di testo (es finalità altro)
Dependent Fields
As indicated, there are some different types of fields: optional, mandatory and dependent. Mandatory and optional are clear: they correspond to, respectively, mandatory fields without which the submission is invalid, and optional fields that are non-compulsory but useful for a particular purpose.
Dependent fields could be mandatory or blank fields depending on value/s from another field/s. There are 6 dependent fields in the submission, they are: Place of birth, NHP Source and NHP Generation depend on Species, Genetic Status and Re-use.
Place of birth must be completed if and only if Re-use is set to "No" and, in Species, is selected a non-NHP (values outside the range A19~A25). On the other hand, NHP Source and NHP Generation must be completed only if NHP is selected in Species (values inside the range A19~A25). For better understanding, below you can see a dependency diagram and table for these fields.
The next 2 dependent fields are Testing by Legislation and Legislative Requirements. These 2 fields depend on the Purpose field. If the Purpose value does not start with code PR, leave these 2 dependent fields blank. If Purpose value starts with code PR, you have to select values for these 2 fields from the dropdown list.
There is one special field: Purpose. Purpose is mandatory in any case but its list can change depending on your selection in Creation of a new GL:
If you choose "Yes" in Creation of a new GL: you can only choose in Purpose field a value inside "Basic research purposes" (codes starting with PB) and "Translational and applied research purposes" (codes starting with PT).
- In other cases you can choose any option from the complete list of purposes.
However, do not worry about this, because excel will create a purpose list dynamically. So, you will not be able to introduce a bad value for purpose.
Finally, if Genetic Status is set to Not Genetically altered it is not possible to chose Purpose PG43, namely Mainteinance of Colonies of established genetically altered animals not used in other procedures
Torna alla homepage del manuale.
